
Selecting the Right CDMO: Key Considerations for Pharmaceutical Companies
Contract Development and Manufacturing Organizations (CDMOs) play a vital role
Welcome to Varaa CMC Consulting
Your Bioprocess Development Partner
We specialize in process development, tech transfer, and regulatory support, ensuring seamless execution for preclinical to clinical stage biotechnology companies.


Vaara CMC Consulting is a trusted partner in bioprocess development, offering tailored solutions for biotech and pharmaceutical companies. With years of industry experience in large molecules such as mAbs, bispecifics, Fc-fusions, and viral vectors, we specialize in optimizing processes, providing manufacturing support, and ensuring quality/regulatory compliance.
Expertise, innovation, and comprehensive support to drive bioprocess success from development to commercialization
Extensive Industry Expertise
Leveraging years of experience to optimize bioprocess development, and Chemistry, Manufacturing, and Controls (CMC).
Our collaborative approach enables speed, flexibility, and capital efficiency.
Providing seamless guidance from development to commercialization.
Navigating CMC and milestones in the biopharmacetucial industry can be complex, but our expertise ensures a smooth and compliant drug substance/drug product process. We provide comprehensive support in regulatory documentation, process validation, and GMP adherence to help businesses meet FDA, EMA, and ICH guidelines. Our team works closely with you to streamline approvals, enhance quality assurance, and maintain compliance with global industry standards. Whether you need assistance with process development, help with CDMO selection, or hands-on lab support we are here to support your success.
Enhancing upstream/downstream processes, scale-up strategies, and high-throughput development.
Assisting with RFPs, technology transfer, and document author and review.
Establishing strategic phase appropriate process strategies.
Augment your staff to provide experimental strategy, data analysis, and training support.
At Vaara CMC consulting, we provide a full range of services to enhance efficiency, scalability, and regulatory compliance in bioprocess development. Our expertise ensures seamless project execution for biotech and pharmaceutical companies
Improving upstream/downstream processes, scale-up strategies, and high-throughput development
Supporting CDMO selection, RFPs, tech transfer, and batch record reviews.
Providing lab buildout, equipment evaluation, and hands-on training.
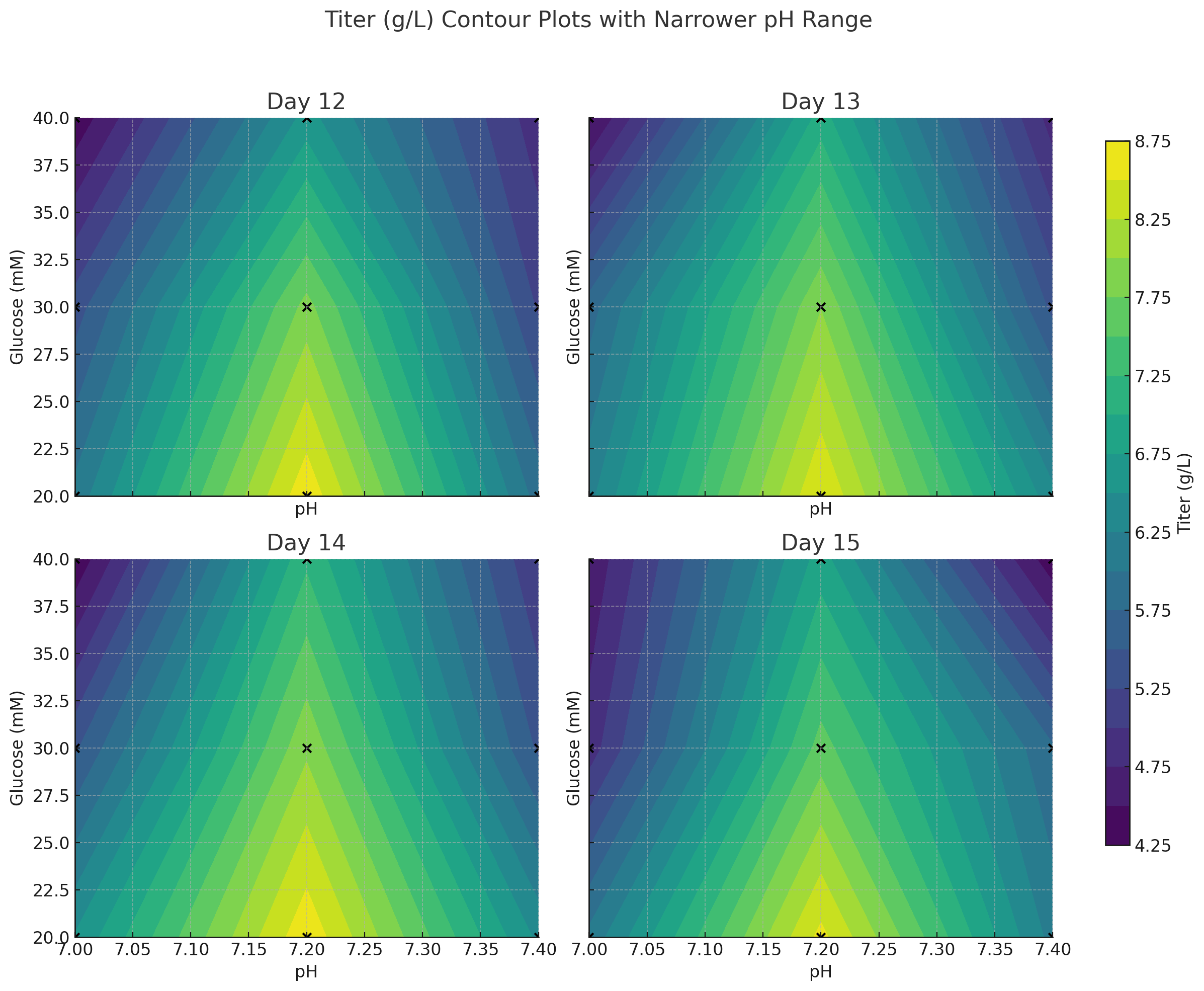
Optimizing Your Bioprocessing Strategy
Our process development services focus on:


Seamless Transition from Development to Manufacturing
Navigating CDMO selection and technology transfer can be complex.
Helping you identify the right manufacturing partner
Ensuring smooth knowledge and process transfer.
Assessing compliance with regulatory standards
Direct support during execution
Driving Success through Strategic Solutions
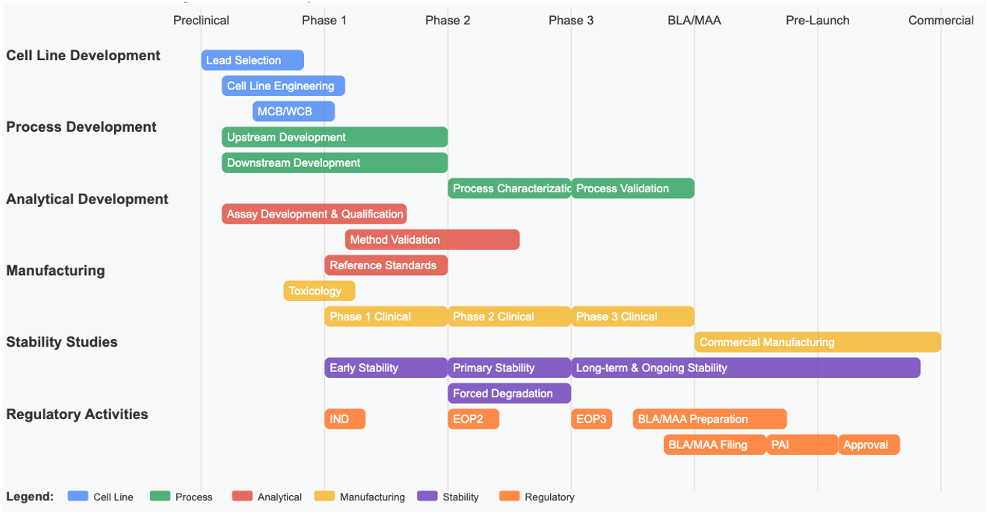
Our Approach to CMC Strategy
Empowering Innovation Through Experimental Excellence
Get it right the first time with hands on support for critical experiments, lab buildouts, equipment procurement and staff training.
Navigating regulatory requirements in the pharmaceutical and chemical industries can be complex, but our expertise ensures a smooth and compliant process. We provide comprehensive support in regulatory documentation, process validation, and GMP adherence to help businesses meet FDA, EMA, and ICH guidelines. Our team works closely with you to streamline approvals, enhance quality assurance, and maintain compliance with global industry standards. Whether you need assistance with regulatory submissions, audits, or compliance assessments, we are here to support your success.
We create detailed Module 3 (CMC) dossiers to ensure smooth regulatory approvals worldwide.
Our experts validate manufacturing processes to meet FDA, EMA, and ICH standards.
We ensure GMP compliance and robust quality control for product safety and consistency.
From audits to submission guidance, we help you stay compliant with evolving regulations.
Enhance efficiency and scalability by refining manufacturing processes, optimizing formulations, and ensuring consistent product quality.

Equip your team with essential knowledge of Good Manufacturing Practices (GMP) and global regulatory standards to ensure compliance and quality assurance.

Ensure the selection of high-quality, cost-effective equipment through thorough assessment, validation, and compliance with industry standards.

Equip your team with essential knowledge of Good Manufacturing Practices (GMP) and global regulatory standards to ensure compliance and quality assurance.
Ensure the selection of high-quality, cost-effective equipment through thorough assessment, validation, and compliance with industry standards.


Enhance efficiency and scalability by refining manufacturing processes, optimizing formulations, and ensuring consistent product quality.


Contract Development and Manufacturing Organizations (CDMOs) play a vital role

In pharmaceutical manufacturing, ensuring a consistent and reproducible production process

In pharmaceutical manufacturing, ensuring a consistent and reproducible process is

Good Manufacturing Practices (GMP) form the backbone of pharmaceutical quality
We provide expert guidance in regulatory compliance, process optimization, and GMP training to support your success.
We provide regulatory consulting, process validation, GMP training, and equipment evaluation to ensure compliance and efficiency.
Our experts help businesses align with FDA, EMA, and ICH guidelines through structured assessments, audits, and documentation support.
Process validation ensures that pharmaceutical manufacturing processes consistently produce safe, high-quality, and reproducible products.
We offer specialized training in regulatory compliance, GMP adherence, process development, and equipment selection.
Our experts analyze critical process parameters (CPPs) and suggest improvements to enhance efficiency and reduce errors.
GMP (Good Manufacturing Practices) certification ensures that pharmaceutical and biotech companies follow strict quality control measures.
We assist in evaluating cost-effective, high-quality laboratory and production equipment that meets industry and regulatory standards.
Regulatory approval ensures that drugs meet safety, efficacy, and quality requirements before reaching the market, avoiding legal risks.
CMC (Chemistry, Manufacturing, and Controls) focuses on maintaining the quality, stability, and consistency of drug products.
You can reach us via our website, email, or phone to discuss your project needs and regulatory compliance support.
At Varaa CMC Consulting, we understand the complexities of CMC (Chemistry, Manufacturing, and Controls) processes and regulatory compliance. We are dedicated to providing strategic guidance, tailored solutions, and expert insights to help you navigate challenges in process development, CDMO management, CMC strategy, and lab support.
Whether you’re a pharmaceutical company, biotech startup, or an established industry leader, our experts are here to offer consultation, optimize your workflows, and ensure regulatory success.